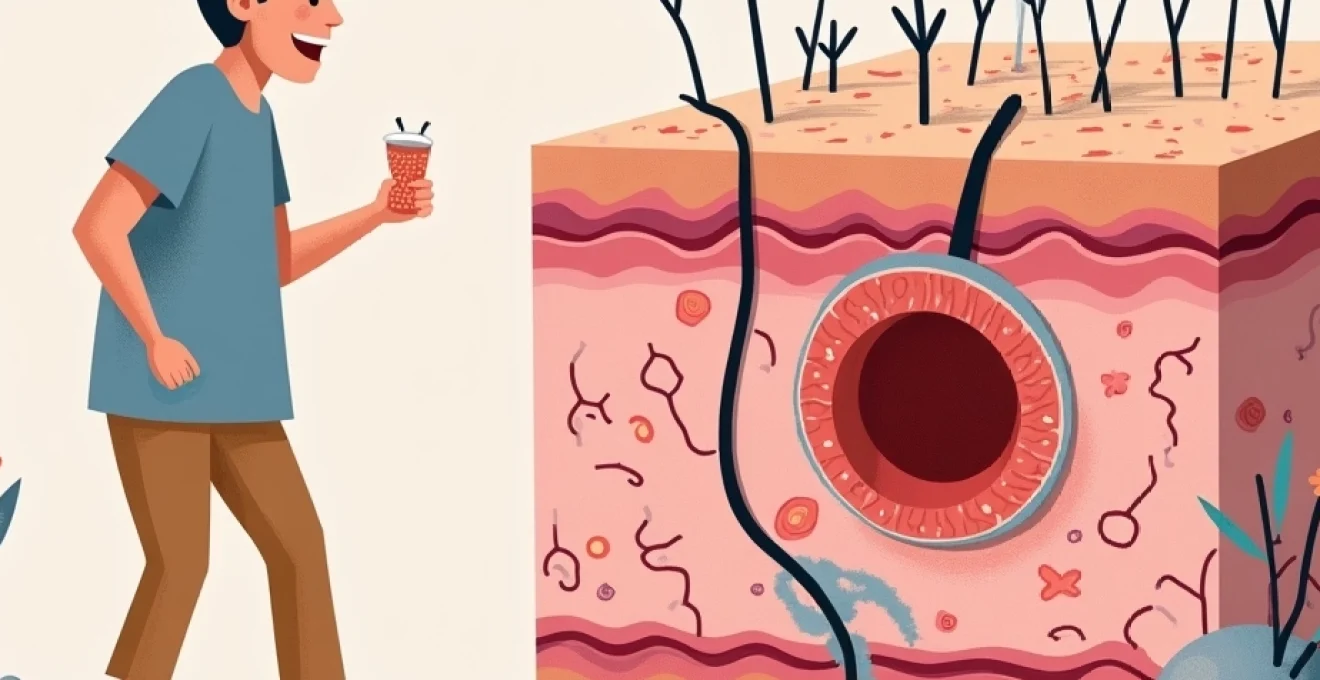
That familiar, pulsating pain radiating from a swollen pimple represents far more than simple skin irritation. The throbbing sensation you experience stems from a complex cascade of inflammatory processes occurring deep within your skin’s layers. When acne lesions develop, your body launches an intricate immune response involving bacterial colonisation, vascular changes, and neurological activation that creates the characteristic pain associated with inflamed spots.
Understanding why pimples throb requires examining the sophisticated biological mechanisms at work beneath the surface. From the initial bacterial invasion to the final stages of tissue repair, each phase contributes to the uncomfortable sensations that make touching or even thinking about your breakout unbearable. This knowledge proves invaluable for anyone seeking effective treatment approaches and pain management strategies.
Inflammatory cascade response in acne vulgaris pathogenesis
The throbbing pain associated with acne lesions originates from a sophisticated inflammatory cascade that begins when comedones become infected with pathogenic bacteria. This complex biological response involves multiple cellular and molecular mechanisms working simultaneously to address the perceived threat within your follicular environment.
Propionibacterium acnes colonisation and biofilm formation
Cutibacterium acnes (formerly Propionibacterium acnes) represents the primary bacterial culprit responsible for transforming simple comedones into painful, throbbing lesions. These anaerobic bacteria thrive in the oxygen-poor environment created by sebum and keratin plugs within blocked follicles. Once established, they form protective biofilms that shield them from both topical treatments and immune system responses.
The biofilm formation process significantly amplifies inflammatory responses by creating persistent bacterial reservoirs that continuously release inflammatory mediators. These bacterial communities produce enzymes such as lipase and hyaluronidase, which break down sebum into free fatty acids that irritate surrounding tissues. The resulting tissue damage triggers your immune system to mount an aggressive response, leading to the characteristic swelling and throbbing associated with inflamed acne lesions.
Toll-like receptor activation and cytokine release mechanisms
Your skin’s innate immune system recognises bacterial invasion through specialised pattern recognition receptors called Toll-like receptors (TLRs). TLR2 and TLR4 play particularly crucial roles in detecting C. acnes components, triggering immediate inflammatory responses upon activation. This recognition system functions like a sophisticated alarm network, instantly alerting surrounding cells to the bacterial threat.
Following TLR activation, keratinocytes and sebocytes begin producing pro-inflammatory cytokines including interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), and interleukin-8 (IL-8). These chemical messengers amplify the inflammatory response by recruiting additional immune cells and promoting vascular changes that contribute directly to the throbbing sensation you experience.
Neutrophil infiltration and chemotactic factor production
The release of chemotactic factors, particularly IL-8 and leukotriene B4, creates a powerful attraction signal for neutrophils circulating in nearby blood vessels. These first-responder immune cells rapidly migrate towards infected follicles, crossing vascular barriers and accumulating in perifollicular tissues. The sheer volume of neutrophil infiltration contributes significantly to tissue swelling and pressure buildup.
Neutrophils employ various antimicrobial strategies including degranulation and neutrophil extracellular trap (NET) formation to combat bacterial infection. However, these defensive mechanisms also release damaging enzymes and reactive oxygen species that inadvertently harm surrounding healthy tissue, perpetuating inflammation and intensifying pain perception.
Complement system activation in comedonal inflammation
The complement system provides another layer of immune defence that becomes activated during severe acne inflammation. C. acnes can directly activate complement pathways, leading to the formation of membrane attack complexes that damage bacterial cell walls. This process generates complement fragments C3a and C5a, which function as potent inflammatory mediators.
These complement fragments increase vascular permeability, promote mast cell degranulation, and attract additional inflammatory cells to the infection site. The resulting tissue changes contribute directly to the pressure buildup and throbbing sensations characteristic of inflammatory acne lesions.
Prostaglandin E2 synthesis and pain receptor sensitisation
Cyclooxygenase-2 (COX-2) upregulation within inflamed follicular tissues leads to increased prostaglandin E2 (PGE2) synthesis, a critical mediator of both inflammation and pain perception. PGE2 directly sensitises nociceptors (pain receptors) in the surrounding dermis, lowering their activation threshold and amplifying pain signals transmitted to your central nervous system.
The synthesis of prostaglandin E2 represents a crucial turning point where inflammation transitions from tissue defence to pain generation, explaining why anti-inflammatory medications often provide effective relief from acne-related discomfort.
Additionally, PGE2 promotes vasodilation and increases vascular permeability, contributing to tissue swelling that creates mechanical pressure on surrounding nerve endings. This dual mechanism – direct receptor sensitisation combined with mechanical compression – explains why inflamed pimples exhibit such intense, persistent throbbing sensations.
Vascular dilation and increased capillary permeability
The characteristic redness and swelling surrounding inflamed acne lesions result from dramatic changes in local blood vessel behaviour. These vascular alterations serve essential functions in immune defence but simultaneously create the physical conditions responsible for throbbing pain. Understanding these mechanisms reveals why certain treatments prove more effective than others for managing acne-related discomfort.
Histamine-mediated vasodilation in perifollicular tissues
Mast cells residing in perifollicular connective tissue become activated by bacterial toxins and inflammatory cytokines, leading to rapid histamine degranulation. This histamine release causes immediate smooth muscle relaxation in surrounding arterioles and capillaries, dramatically increasing blood flow to the affected area. The enhanced circulation creates the visible redness and warmth characteristic of inflamed lesions.
The increased blood volume within dilated vessels creates elevated hydrostatic pressure that forces plasma components through vessel walls into surrounding tissues. This process, known as transudation, contributes significantly to the tissue swelling that generates mechanical pressure on pain receptors, resulting in the pulsating sensation synchronised with your heartbeat.
Nitric oxide synthase expression and endothelial function
Inflammatory cytokines, particularly TNF-α and IL-1β, stimulate increased expression of inducible nitric oxide synthase (iNOS) within vascular endothelial cells surrounding infected follicles. This enzyme produces nitric oxide, a potent vasodilator that maintains prolonged blood vessel dilation even after initial histamine effects subside.
The sustained vasodilation mediated by nitric oxide explains why acne lesions remain red and swollen for extended periods, often days or weeks after initial bacterial colonisation. This persistent vascular dilation maintains elevated tissue pressure that continues generating throbbing sensations throughout the inflammatory process.
Bradykinin release and vascular smooth muscle relaxation
The kallikrein-kinin system becomes activated during tissue injury associated with severe acne inflammation, leading to bradykinin generation from plasma kininogen precursors. Bradykinin represents one of the most potent naturally occurring vasodilators and pain mediators, directly stimulating B2 receptors on vascular smooth muscle cells and sensory nerve endings.
Bradykinin’s dual action – causing both vasodilation and direct pain receptor activation – makes it a particularly significant contributor to acne-related throbbing. The peptide also increases vascular permeability by creating gaps between endothelial cells, allowing inflammatory mediators and immune cells to enter perifollicular tissues more readily.
Plasma protein extravasation and tissue oedema formation
The combination of increased vascular permeability and elevated hydrostatic pressure forces plasma proteins, particularly albumin and fibrinogen, out of blood vessels and into surrounding dermal tissues. This protein extravasation significantly increases tissue osmotic pressure, drawing additional water into the affected area and creating substantial tissue swelling.
The resulting oedema formation creates a self-perpetuating cycle where increased tissue volume generates greater pressure on surrounding structures, including nerve endings and adjacent follicles. This mechanical compression explains why inflamed acne lesions often feel tender to touch and why the throbbing sensation intensifies with physical pressure or movement.
Mechanoreceptor activation and nociceptive pathway stimulation
The throbbing pain experienced with inflamed acne lesions results from activation of specialised sensory receptors embedded throughout dermal tissues. These sophisticated detection systems respond to both chemical inflammatory mediators and mechanical pressure changes, transmitting pain signals through complex neural pathways to your central nervous system. The integration of these signals creates the characteristic pulsating discomfort that makes touching or manipulating acne lesions so uncomfortable.
Mechanoreceptors within perifollicular tissues become hypersensitive during inflammation due to the combined effects of tissue swelling and inflammatory mediator exposure. Nociceptors , specifically designed to detect potentially harmful stimuli, respond dramatically to the chemical soup created by bacterial infection and immune response. The resulting neural activity generates both constant background pain and the rhythmic throbbing sensation that corresponds with vascular pulsations.
The transmission of pain signals from inflamed acne lesions involves multiple classes of sensory fibres with varying conduction velocities. A-delta fibres carry sharp, localised pain signals rapidly to the spinal cord, while C-fibres transmit slower, more diffuse burning and throbbing sensations. This dual innervation explains why you experience both immediate sharp pain when touching an inflamed lesion and the persistent, deeper throbbing that continues even without direct contact.
The sophisticated pain transmission system surrounding hair follicles evolved to protect these structures from damage, but during severe inflammation, this protective mechanism becomes hyperactive, generating pain signals disproportionate to the actual tissue damage present.
Sensitisation of nociceptive pathways occurs through multiple mechanisms during acne inflammation. Inflammatory mediators such as substance P, calcitonin gene-related peptide (CGRP), and nerve growth factor (NGF) directly modify sensory nerve function, lowering activation thresholds and increasing signal transmission frequency. This neuroplasticity explains why even gentle pressure on inflamed lesions produces intense discomfort and why the affected area remains hypersensitive even after visible inflammation subsides.
Sebaceous gland hyperactivity and follicular pressure dynamics
Sebaceous gland dysfunction plays a fundamental role in creating the pressure dynamics that contribute to acne-related throbbing sensations. When these oil-producing structures become hyperactive due to hormonal stimulation or inflammatory signalling, they generate excessive sebum that combines with desquamated keratinocytes to create dense, cohesive plugs within hair follicles. This process creates a closed system under increasing pressure as continued sebum production has nowhere to escape.
The mechanical properties of sebum change significantly during inflammation, becoming more viscous and adhesive due to oxidative processes and bacterial enzyme activity. This altered sebum consistency makes follicular clearance more difficult while simultaneously increasing the structural integrity of comedonal plugs. The result is a progressively increasing pressure system that stretches follicular walls and compresses surrounding nerve endings, generating continuous mechanical stimulation of pain receptors.
Follicular distension reaches critical levels when internal pressure overcomes the structural integrity of follicular walls, leading to rupture and spillage of contents into surrounding dermal tissues. This rupture event represents a catastrophic release of pressure that immediately triggers intense inflammatory responses and severe pain. The sudden pressure change explains why some individuals report experiencing sharp, shooting pains just before a deep pimple becomes more inflamed and painful.
Intrafollicular pressure measurements during active acne inflammation demonstrate pressures that can exceed normal tissue hydrostatic pressure by several-fold. These elevated pressures create constant mechanical stress on surrounding tissues, contributing to the persistent aching and throbbing sensations characteristic of deep, cystic acne lesions. The pressure also impairs local blood circulation, creating areas of relative hypoxia that further stimulate inflammatory mediator production.
The relationship between sebaceous gland activity and follicular pressure involves complex feedback mechanisms influenced by local inflammatory conditions. Pro-inflammatory cytokines such as IL-1α directly stimulate sebaceous cell proliferation and lipid synthesis, creating a positive feedback loop where inflammation increases sebum production, which in turn maintains and amplifies inflammatory responses. This self-perpetuating cycle explains why some acne lesions persist for extended periods and why the associated pain can be so difficult to resolve without targeted intervention.
Hormonal modulation of pain perception in acne lesions
Hormonal fluctuations significantly influence both acne development and the intensity of associated pain perception through multiple interconnected mechanisms. Androgens, particularly dihydrotestosterone (DHT), not only stimulate sebaceous gland hyperactivity but also modulate inflammatory responses and pain sensitivity within follicular tissues. This hormonal influence explains why acne-related pain often varies with menstrual cycles, puberty, and other periods of hormonal change.
Oestrogen receptors present within sebaceous glands and surrounding dermal tissues provide another layer of hormonal modulation that affects both inflammation severity and pain perception. During periods of oestrogen dominance, anti-inflammatory effects tend to predominate, potentially reducing both lesion severity and associated discomfort. Conversely, oestrogen withdrawal, such as occurs during the luteal phase of menstrual cycles, can intensify inflammatory responses and increase pain sensitivity.
Cortisol levels significantly impact the inflammatory cascade associated with acne development and the resulting pain experience. Chronic stress leads to dysregulated cortisol production, which can paradoxically increase inflammation despite cortisol’s typical anti-inflammatory properties. This dysregulation occurs through altered glucocorticoid receptor sensitivity and modified cytokine production patterns that favour pro-inflammatory responses.
The interaction between stress hormones and pain perception creates a complex feedback loop where acne-related discomfort increases psychological stress, which in turn elevates cortisol levels and potentially worsens both inflammation and pain sensitivity. Breaking this cycle often requires comprehensive approaches that address both the physical inflammatory process and the psychological stress response.
Growth hormone and insulin-like growth factor-1 (IGF-1) represent additional hormonal influences that affect both acne severity and pain perception. These anabolic hormones stimulate keratinocyte proliferation and sebaceous gland development while also influencing inflammatory cell function and pain receptor sensitivity. Dietary factors that influence IGF-1 levels, such as dairy consumption and high-glycemic foods, may therefore indirectly affect the intensity of acne-related pain through these hormonal pathways.